Cryopreparation
Fixation and dehydration at ambient temperatures leads to artifacts due to shrinkage, extraction, aggregation and redistribution of biological material. Cryopreparation techniques aim at avoiding such artifacts.
Native or chemically fixed samples are rapidly frozen at ambient pressure (e.g. by plunge freezing in liquid propane) or at high-pressure with jets of liquid nitrogen in a high-pressure-freezer. Freezing is followed by freeze substitution (FS) in an automated FS unit (AFS) to replace the frozen cell water molecules of the samples by organic solvents and additives at temperatures below the onset of destructive water recrystallization. Subsequent warm-up of the substitution medium ensures low-temperature fixation of the dehydrated sample throughout its volume at the same time. Until recently, the dissolution of “frozen” cell water by organic solvents at temperatures between -80°C to -90°C was time-consuming. Introduction of magnetic force-driven agitation to samples inside the chamber of the AFS by S. Reipert and H. Goldammer (2015) lead to shortening of the protocols from days to a couple of hours. Agitation modules for both the AFS(1) and AFS2 (LEICA Microsystems) are produced by H. Goldammer, Cryomodultech e.U.. They are already used for various applications in EM facilities in Austria and abroad.
FS is followed by resin embedding of cells and tissues for ultrastructural studies, element- and isotope analyses, immunolabeling, or in situ hybridization experiments. For immunolabeling the samples might also be rehydrated, cryoprotected with sucrose and frozen for cryosectioning with a cryo-ultramicrotome. More commonly, this method of cryosectioning (Tokuyasu technique) is applied to samples that have been chemically fixed at ambient temperature, before.
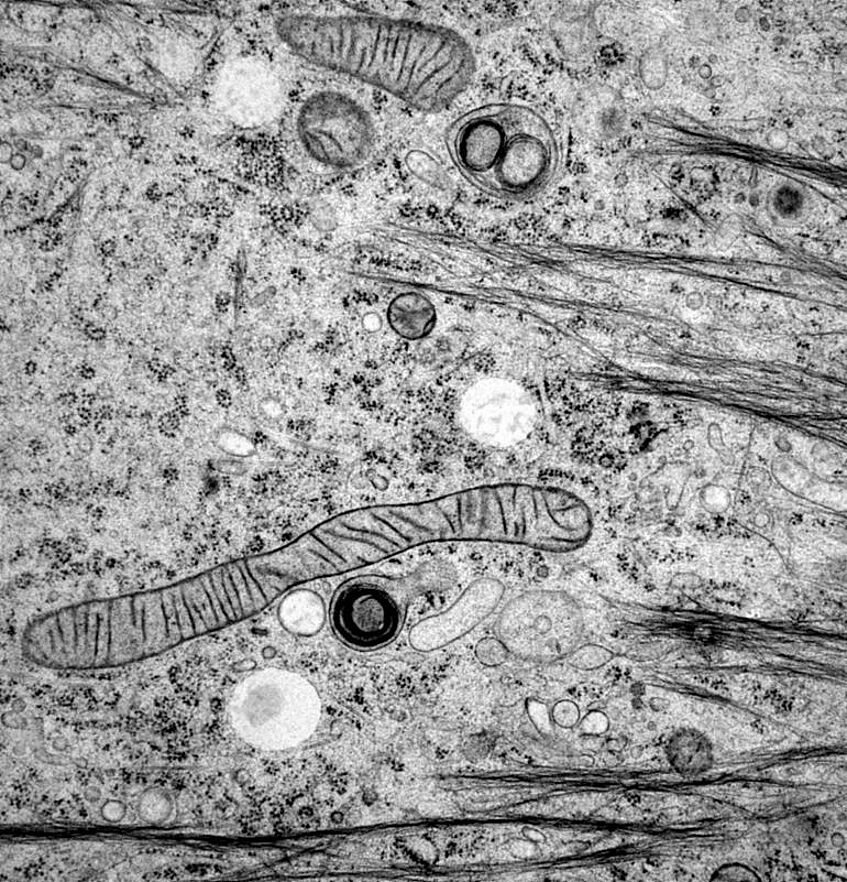
Cytoplasm of a murine keratinocyte, rich in detail after cryopreservation by high-pressure freezing and freeze substitution (Foto: S. Reipert)
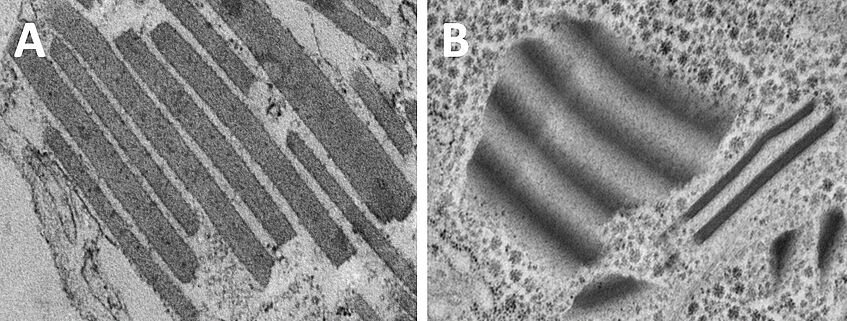
Optical active mesostructures in Artemia franciscana. A) Conventional chemical preparation resulting in destruction of the integrity of the flakes and washes material out of the cytoplasm. B) High-pressure freezing and freeze substitution preserves the essence of the mesostructures. Hollergschwandtner et al. (2017).
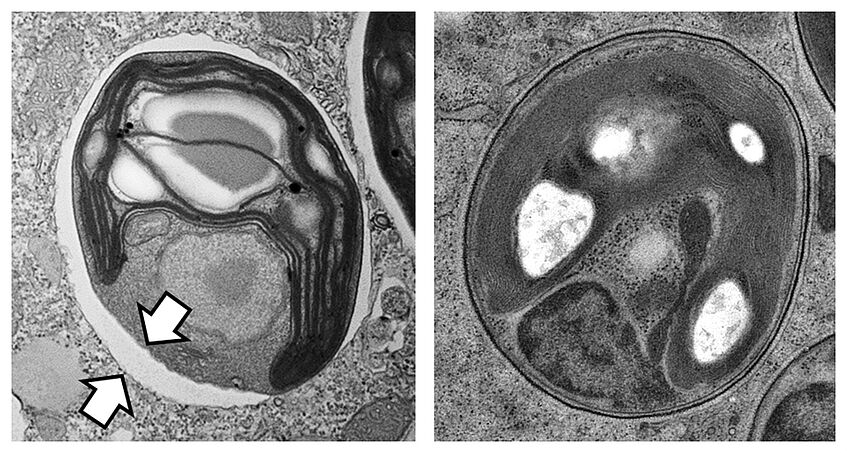
Symbiont/ host interaction between the alga Chlorella and the single-cell organism Paramecium bursaria. The conventionally prepared sample on the left displays a shrinking artifact (arrows) that is prevented by high-pressure freezing and rapid freeze substitution, on the right. Fotos: D. Gruber; S. Reipert
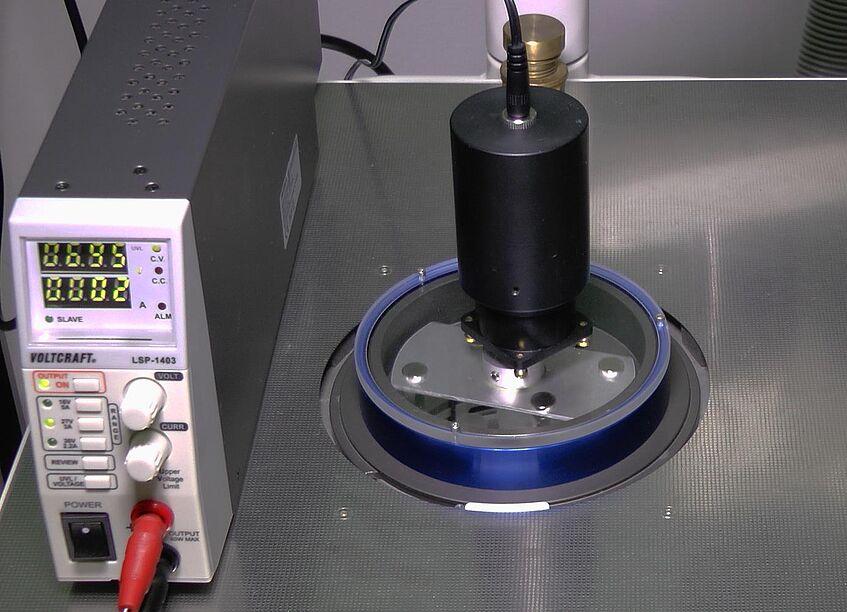
Magnetic force- driven agitation module for accelerated freeze substitution in the AFS 2 (LEICA Microsystems). Austrian patent number AT515423 and PCT application WO2015154118
