Immunolabeling in the transmission electron microscope
Immunolabeling enables the detection and localization of proteins and other organic antigens by using monoclonal or polyclonal antibodies (immunoglobulins). The antibodies are either directly conjugated with markers or they are indirectly recognized by binding to marker-conjugated secondary antibodies. Conjugates such as colloidal gold or enzymes that react with their substrates both lead to an electron-dense labeling that contrasts with the ultrastructures of cells and tissues in the electron microscope.
To succeed with immunolabeling of cells and tissues, considerations regarding the nature, availability and accessibility of the antigen epitopes must be taken into account: Since the antigenicity has to be maintained throughout the sample preparation, compromises in the processing are usually required. The applicant may choose between the immunolabeling prior to resin embedding (pre-embedding labeling) and the labeling of those epitopes that are exposed at the surface of resin sections (postembedding –labeling). The latter is supported by embedding of cells and tissues in epitope-friendly methacrylic resins (LR White, Lowicryls).Moreover, cryosections of aldehyde-fixed, cryoprotected samples might be used for immunolabeling (Tokuyasu method). If required, the immunolabeling concept can be extended towards rapidly cryofixed cells and tissues that are processed by freeze substitution even in absence of epitope-damaging aldehydes.
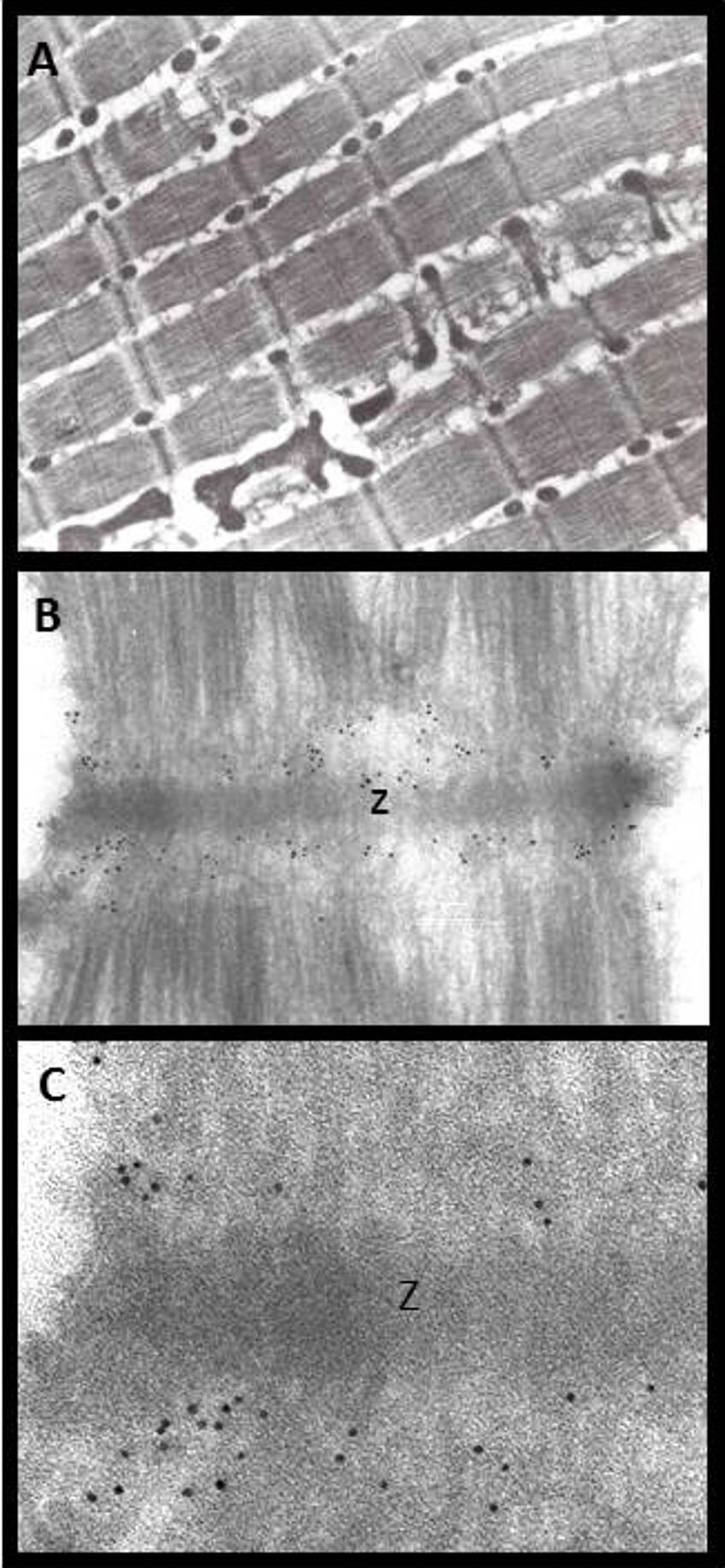
Immunogold labeling of a domain of the giant protein titin. A) Overview of perfusion-fixed, cryosectioned rat muscle (Tokuyasu method). B) Sarcomere with I bands labeled for a titin domain. C) Detail showing 5 nm colloidal gold at I-bands next to a Z-disc. (Primary antibody T12: gift by Dieter Fürst).
